Hematodinium sp (clade B)
Diagnosis
Diagnosis_Genus: The generic characters of Hematodinium are the amphiesmal structure of the pellicle, the condensed and beaded chromatin that forms V-shaped configurations of the chromosomes, the plasmodial nature of the meront, the continuous state of mitotic activity in the nucleus and the type of mitosis exhibited (dinomitosis) (Chatton & Poisson 1931, Newman & Johnson 1975, Cachon & Cachon 1987). Parasitic dinoflagellates responsable for the bitter crab syndrome (BCS, Meyers et al. 1987), named because of the bitter or astringent flavour the parasite imparts to cooked crab meat. Crabs infected by this parasite exhibit a spectrum of disease ranging from asymptomatic carriage to death. Affected hosts undergo dramatic pathological alterations to their organs, tissues, and haemolymph. Respiratory dysfunction results in lethargy, and eventual death.
Diagnosis_Species: SSU and ITS delineate H. perezi (called Clade A) from this undescribed species Clade B (Jensen et al. 2010, Small et al. 2012). Live cycle was well described by Appleton and Vickerman (1998), from isolates obtained from intecfed Norway lobster (Nephrops norvegicus).
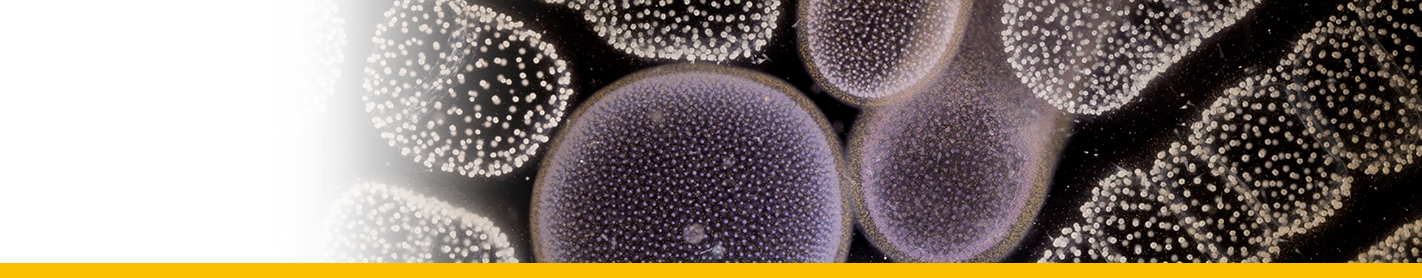
Sporoblasts: Circulating parasites in langoustin hemolymph from late-stage infections. They would eventually give rise by division to flagellated dinospores in the crustacean host. They contained abundant trichocysts and swollen cisternae of the nuclear envelope and endoplasmic reticulum containing filamentous material tentatively identified as flagellar hairs destined for the transverse flagellum of the dinospore. Their nuclei showed highly condensed chromosomes and their surface had the characteristic dinoflagellate amphiesma (alveolate) structure.
Sporogenesis: The sporoblasts increased in number by fission, often forming spherical masses, or sheets of multinucleate cells which eventually broke up to release spore mother cells ; each of which gave rise to 4 or more flagellated dinospores. In the second pattern of development the sporoblasts gave rise to a branched network of filaments (filamentous trophonts) with nuclei scattered along their length, and noticeably attached to the substratum.
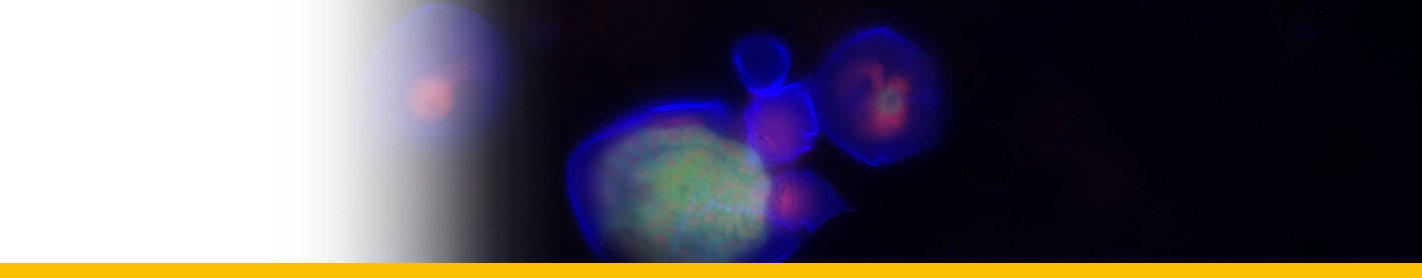
Dinospores: Dinospores produced in vitro (as in vivo) were uninucleate and of 2 types, differing in size and form, termed macrospores (16-20 µm long, 8.5-11.5 µm in width, n=25) and microspores (11-14 µm long, 4.5-6.5 µm in width, n=25). Each spore type had 2 heterodynamic flagella, the anterior flagellum encircling the anterior end of the body in beating and providing the principal propulsive force, the rudderlike posterior flagellum trailing behind. Microspores were intermittently much faster in their movement than macrospores. A striking refractile inclusion in a vacuole at the posterior extremity of the microspore was another distinguishing feature of this spore type. Condensation of the chromosomes in the nucleus was clearly more pronounced in microspores than in macrospores. After a period of about 5 weeks in fresh medium, both types of spore became immotile and settled on the bottom of the culture well. If spore density was adjusted to produce a monolayer, both types of spore were shown to transform into square cells and then
undergo growth and nuclear division to produce multinucleate filaments; no fusion of motile spores was discerned, and each type of spore was found to be capable of germination independent of the other. In the Tanner crabs (Chionoecetes bairdi) from the Sullivan Island area of southeast Alaska, there is no ploidy difference between the two spore types and the vegetative cell, suggesting that the two spore types may not represent separate sexes (Eaton et al. 1991).
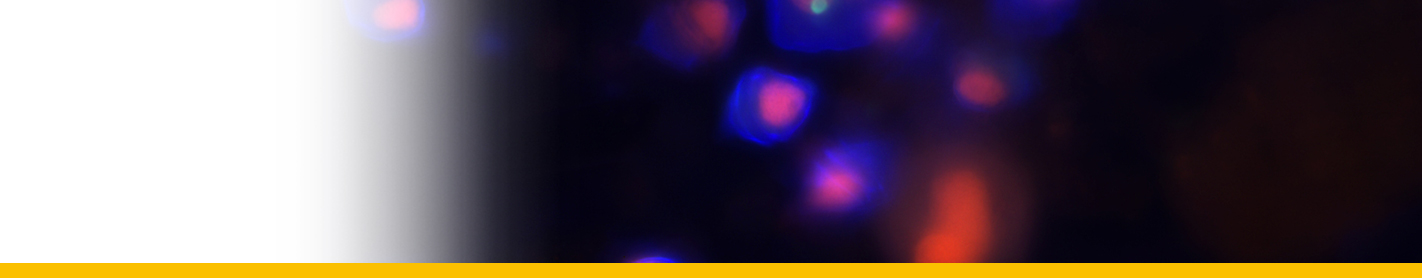
Filamentous trophont: The trophont phase does not contain trichocysts. The unattached 12-50 µm-long filaments produced on germination of the dinospores showed that the majority were uninucleate (50%) or binucleate (43%) and that relatively few (7%) had 3 or more nuclei. they showed lateral flexing and squirming movements. They contain nuclei with extended chromosomes, clustered lipid globules and clear vacuoles, but not trichocysts or flagellar hair vesicles. After growth by increase in length the filaments branched and developed constrictions at branching points, a stage called the 'gorgonlock'. Left for 3 weeks or more, the gorgonlock colonies condensed and became spherical or flattened masses referred to as clump colonies.
Arachnoid trophont: Under certain conditions, the filamentous trophonts, often as gorgonlock colonies, adhere
to the substratum. Then, through progressive nuclear division and growth, and branching and anastomosis of filaments, they formed elaborate syncytial networks (plasmodia) fringed by anchoring spikes. These arachnoid forms expanded
progressively outwards on the base of the culture vessel and coalasced with one another when their spike-like marginal extensions met. In this way they occasionally formed a single plasmodium that covered the floor of the well. The arachnoid trophonts transformed into sporonts which gave rise to dinospores.
Flexing and stretching movements are more evident in the filamentous trophonts of the gorgonlocks colonies than in the isolated filaments. It is possible that this motility confers on the trophont the ability to migrate between tissues.
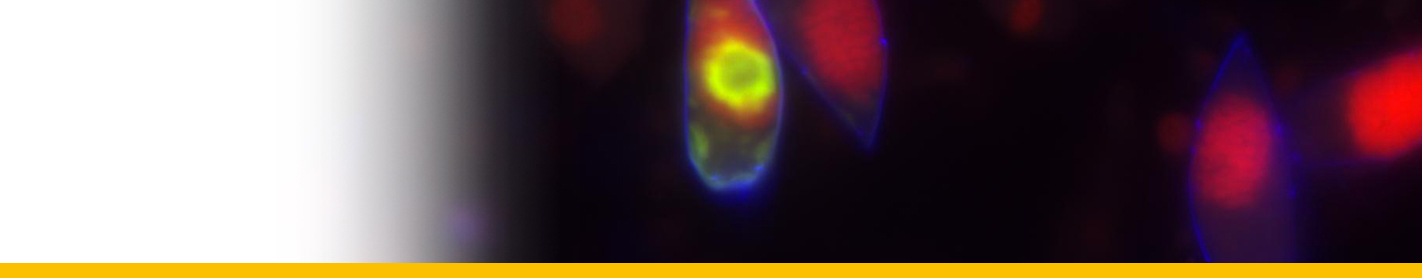
Bacteria-like symbionts have been described in the parasite Clade B infecting the European brown shrimp (Crangon crangon, Stentiford et al. 2012).
Diagnostic: Traditional techniques of disease detection involve macroscopic examination of pleopods that generally result in detection of only relatively advanced infections (Stentiford et al. 2001) or microscopic examination of hemolymph smears. SSU delineates H. perezi (called Clade A, Jensen et al. 2010) from clade B (undescribed Hematodinium). In some host species, advanced infections lead to a discoloration of the carapace from reddish-brown to a reddish ‘cooked’ appearance (e.g. in Nephrops norvegicus and Chionoecetes opilio) and this may be used for detection of the disease (colour method; Meyers et al. 1987, Field et al. 1992). PCR diagnostic assay: the SSU_Univ-F-15: ctcccagtagtcatatgc and SSU_Hemat-F-1487: cctggctcgatagagttg forward primers (5'-3') can be used in complement to the SSU_Hemat-R-1654: ggctgccgtccgaattattcac (5'-3') for a rapid and efficient detection of infected hosts by both Hematodinium clade A and B (Gruebel et al. 2002, Jensen et al. 2010).
Body_macrospores_length: 16-20 µm (in Nephrops norvegicus)
Body_macrospores_wide: 8.5-11.5 µm (in Nephrops norvegicus)
Body_microspores_length: 11-14 µm (in Nephrops norvegicus)
Body_microspores_wide: 4.5-6.5 µm (in Nephrops norvegicus)
Type species
The type species of this genus is H. perezi Chatton & Poisson 1931
Ecology
Substrate_trophont: endozoic
Substrate_trophont: extracellular
Substrate_spores: planktonic
Sociability_trophont: colonial
Salinity: marine
Salinity: variable
Life cycle
Live cycle: In culture the parasite undergoes a characteristic cycle of development. Circulating sporoblasts from the host's haemolymph in vitro generate 2 kinds of flagellated uninucleate dinospore, macrospores and microspores, either of which will, after 5 weeks in fresh medium, germinate to produce multinucleate unattached filamentous trophonts. These trophonts multiply by fragmentation and growth and may be serially subcultured in this form, at 2 week intervals, indefinitely. If not subcultured, the filamentous trophonts give rise to colonies of radiating filaments (`gorgonlocks') which subsequently attach to the substratum to form flattened web-like `arachnoid' multinucleate trophonts. Arachnoid trophonts become arachnoid sporonts when they synthesize trichocysts and flagellar hairs and may give rise to secondary arachnoid sporonts or to dinospores which initiate a new cycle.
Transmission: The most likely pattern of infection of Nephrops would seem to be ingestion of the dinospores, possibly as a result of the suspension-feeding activities of the lobster (Loo, Baden & Ulmestrand, 1993). An alternative route might be via the thin or broken cuticle during moulting. Eaton and coworkers (1991) reported experimental infection of tanner crabs following injection of naturally generated dinospores of their Hematodinium sp.
Phases_alternance: haplontic
Generation: 1-3 months
Resting_stage: spores_asexual
Symbiont: horizontal
Symbiont: horizontal_ingestion
Feeding behaviour
Mode of locomotion
Reference(s)
Attached phylogeny
Observation site(s)
Observation site(s)
HOSTS
SYMBIONTS
| Association with... | Region origin | Name of site | In reference... |
|---|---|---|---|
| Bacteria_like | the Wash |
(2012) Hematodinium sp. and its bacteria-like endosymbiont in European brown shrimp (Crangon crangon). Aquatic Biosystems 8:1-10. |