Hematodinium perezi (clade A)
Diagnosis
Diagnosis_Genus: The generic characters of Hematodinium are the amphiesmal structure of the pellicle, the condensed and beaded chromatin that forms V-shaped configurations of the chromosomes, the plasmodial nature of the meront, the continuous state of mitotic activity in the nucleus and the type of mitosis exhibited (dinomitosis) (Chatton & Poisson 1931, Newman & Johnson 1975, Cachon & Cachon 1987). Parasitic dinoflagellates responsable for the bitter crab syndrome (BCS, Meyers et al. 1987), named because of the bitter or astringent flavour the parasite imparts to cooked crab meat. Crabs infected by this parasite exhibit a spectrum of disease ranging from asymptomatic carriage to death. Affected hosts undergo dramatic pathological alterations to their organs, tissues, and haemolymph. Respiratory dysfunction results in lethargy, and eventual death.
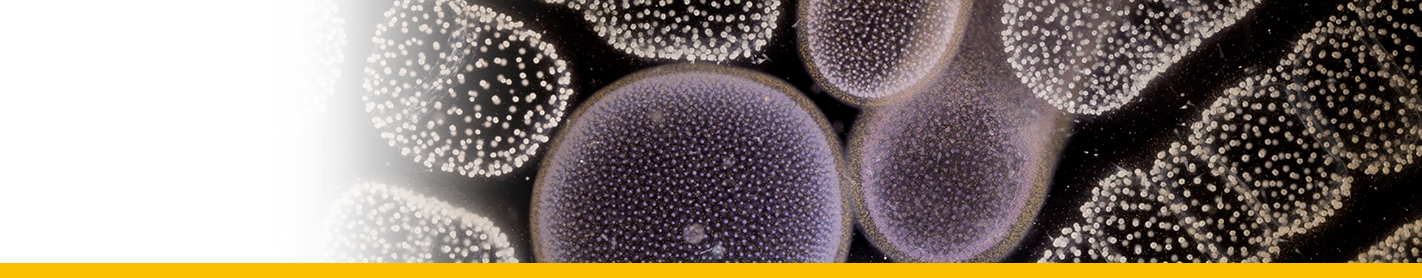
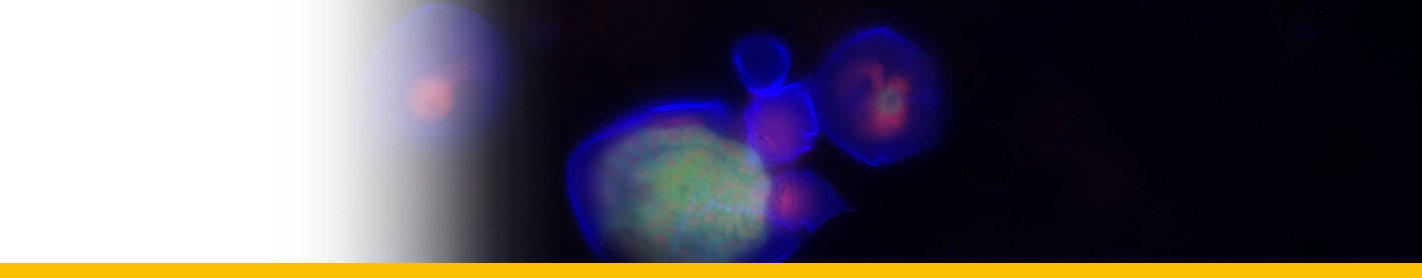
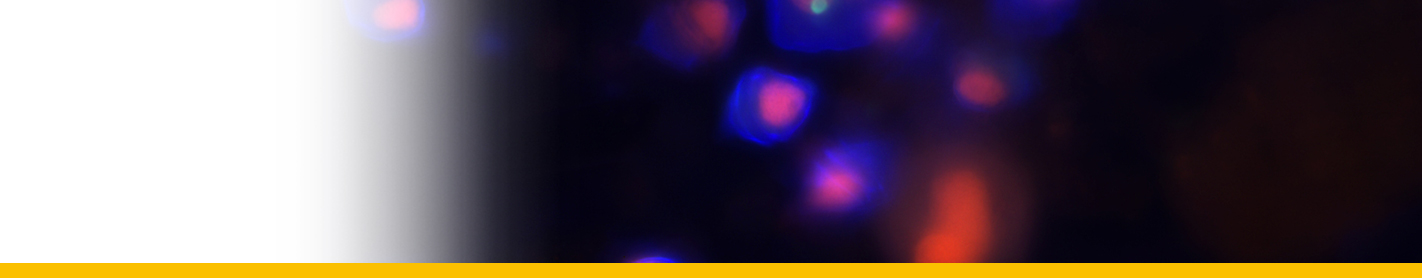
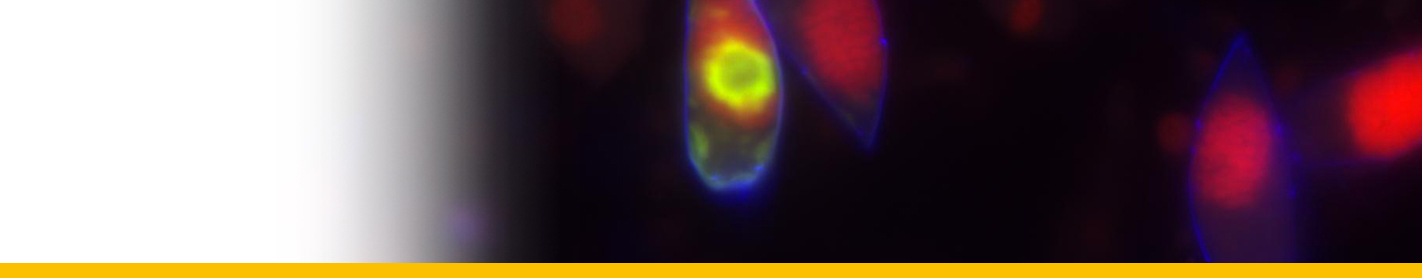
Diagnosis_Species: SSU delineates H. perezi (called Clade A, Jensen et al. 2010) from clade B (undescribed Hematodinium). ITS region sequences allowed the discrimination of three distinct H. perezi clade A genotypes (based upon SSU and ITS, Small et al. 2012), infecting the following hosts L. depurator (genotype I), P. trituberculatus and S. serrata (genotype II), and C. sapidus (genotype III). The subtly different H. perezi ITS1 sequences presented in genotypes I, II, and III, may arising from presumably rapidly evolving due to a host switch, and, in particular, the closer affinity that genotype II in hosts from China has with genotype I in L. depurator from Europe, rather than with genotype III in C. sapidus from the United States. In hemolymph, the parasite has different shape, from unnucleate cell to globulous morphotypes. Uninucleate trophont ranged from 9 to 15 μm in diameter; while multinucleate sporonts or filamentous trophonts were normally 3–5 times larger. Young elongated trophont, often bifurcated, are motile (by torsion). Nuclei continuously divide, and have V-shaped chromosomes (5 in total), similarly to chromosomes described in Syndinium. The parasites are identified by their nuclei containing condensed chromatin, the alveolar membrane, cytoplasmic trichocysts, and vacuoles, and, when observed, athecate dinospores. Dinospores, which are thought to be the infective stage, are detectable for only a short time (i.e. up to 7 d) after being released into the seawater from infected crabs, this short survival time may limit its transmission potential (Li et al. 2010). Two types of dinospores are produced, but a trophont produces only one type of spore (Li et al. 2011). In C. sapidus, the macro-dinospores ranged in size from 11 to 17 μm (14·3±1·6 μm, n=25) excluding measurement of the flagella. The micro-dinospores ranged in size from 6 to 12 μm (9·2±2·4 μm, n=25). A more complex live cycle was discovered from isolates (see Live cycle)
Diagnostic: Traditional techniques of disease detection involve macroscopic examination of pleopods that generally result in detection of only relatively advanced infections (Stentiford et al. 2001) or microscopic examination of hemolymph smears. SSU delineates H. perezi (called Clade A, Jensen et al. 2010) from clade B (undescribed Hematodinium). ITS region sequences allowed the discrimination of three distinct H. perezi clade A genotypes (based upon SSU and ITS, Small et al. 2012), infecting the following hosts L. depurator (genotype I), P. trituberculatus and S. serrata (genotype II), and C. sapidus (genotype III). The subtly different H. perezi ITS1 sequences presented in genotypes I, II, and III, may arising from presumably rapidly evolving due to a host switch, and, in particular, the closer affinity that genotype II in hosts from China has with genotype I in L. depurator from Europe, rather than with genotype III in C. sapidus from the United States. PCR diagnostic assay: the SSU_Univ-F-15: ctcccagtagtcatatgc and SSU_Hemat-F-1487: cctggctcgatagagttg forward primers (5'-3') can be used in complement to the SSU_Hemat-R-1654: ggctgccgtccgaattattcac (5'-3') for a rapid and efficient detection of infected hosts by both Hematodinium clade A and B (Gruebel et al. 2002, Jensen et al. 2010).
Body_macrospores: 11-17 μm (In C. sapidus)
Body_microspores: 6-12 μm (In C. sapidus)
Etymology
Species dedicated to prof. Ch Pérez, who first discovered this parasite in 1905 from infected carcinus maenas from Arcachon (France).
Type species
This is the type species of the genus.
Type illustration / Type locality / Type specimen
Type host: Liocarcinus depurator and Carcinus maenas from France (different sites), Chatton & Poisson (1931)
Ecology
In the coastal bays of Maryland and Virginia, prevalence of H. perezi infecting the blue crab Callinectes sapidus followed a seasonal pattern, with a sharp peak in late autumn (Messik et al. 2000). However, its presence was consistently highest in winter season samples in Gandy et al. 2015.
Infections of blue crab were significantly more prevalent in crabs measuring less than 30 mm carapace width; host sex did not influence prevalence. Prevalences were highest in crabs collected from salinities of 26 to 30‰; no infected crabs were found in salinities below 11‰. Intensity of infection did not vary among crab sizes, molt stages, or sexes (Messik et al. 2000).
Infected crabs suffered significantly higher predation than uninfected crabs when tethered in the field. Similarly, infected juvenile crabs were preyed upon significantly more often than uninfected conspecifics when exposed to a predatory adult crab in laboratory experiments. Laboratory experiments also revealed that the behavior of infected and uninfected crabs differed in ways that affected their risk of predation (Butler IV et al. 2014).
The use of microsatellite markers from H. perezi (III) indicated that simultaneous infections with multiple genetic types in a single-host individual were common and observed in 42 % of the samples. The remaining 58 % of samples had a single allele per locus at all eight polymorphic loci suggesting that the life history stages of the parasite in the host hemolymph are likely haploid.
Substrate_trophont: endozoic
Substrate_trophont: extracellular
Substrate_spores: planktonic
Sociability_trophont: colonial
Sociability_trophont: sometime gregarious
Salinity: marine
Salinity: variable
Life cycle
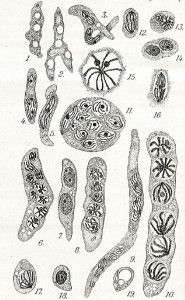
Live cycle: The life cycle of Hematodinium perezi (Clade A, genotype III) in blue crabs is well described by Li et al. 2011 from isolates in culture:
Filamentous trophonts, or vermiform plasmodia, were observed in circulating haemolymph or tissues of blue crabs with very light Hematodinium infections. This stage is easily distinguishable from host haemocytes as the trophont is motile, vermiform, and up to 100 μm in length in the haemolymph. In haemolymph and in culture the filamentous trophonts displayed slow motility such as lateral stretching, flexing and writhing movements, with cytoplasmic streaming inside the cell. The filamentous trophonts were uninucleate, binucleate or multinucleate. filamentous trophonts multiplied by what appeared to be budding wherein they developed constriction points and separated at the constriction. In cultures, this stage was very brief, usually only occurring over 1–3 days. They also underwent a division process resembling merogony (cf. segmentation) with rapid asexual division that led to the next stage, the amoeboid trophonts.
Amoeboid trophonts were the most common stage observed in haemolymph or tissues of crabs with light to moderate infections. Unlike filamentous trophonts, amoeboid trophonts displayed little to no motility in crab haemolymph or in vitro culture. In haemolymph this stage often possessed a single short lobopod or irregular margin. In culture the cells were often more rounded and globular, less amoeboid in shape. Uninucleate, binucleate or multinucleate cells of this stage have been observed in both diseased crabs and in in vitro cultures. Amoeboid trophonts were observed to undergo binary fission to produce more trophonts. In culture, amoeboid trophonts maintained their oval or spherical shape for 1–3 days, and then adhered to the bottom of the culture flasks or plates, and developed into arachnoid trophonts.
The arachonoid trophont stage was the primary stage for rapid amplification of the parasite in culture. This stage was not observed circulating in the haemolymph of infected crabs as it is apparently a tissue phase of the parasite. Shortly after the amoeboid trophonts adhered to the culture flasks or plates, they began developing pseudopodial branches with anastomosis of the pseudopods into an elaborate syncytial network fringed by adherent reticulopodialike pseudopods. The centroids of these trophonts were clearly undergoing rapid division to produce an apparent multicellular stage. Through continuous asexual division, these arachnoid forms expanded progressively outward over the substrate of the culture vessels. Surprisingly, isolated syncytial networks that came into contact with each other merged to form a larger syncytial network. The arachnoid trophonts in these cultures often possessed enlarged centroid cells, reminiscent of germinative cells that were undergoing apparent budding on the periphery of the cell. Asexual division in these centroid cells rapidly led to a large mass of cells, the whole complex termed an arachnoid sporont. The arachnoid sporont was characterized by the central area becoming a cellular mass, with the syncytial network gradually contracting and vanishing within the expansion of the central mass of cells. Approximately 4–6 weeks after isolation, arachnoid trophonts developed into arachnoid sporonts.
Sporoblasts that detached from the arachnoid sporonts formed cellular aggregates or eventually dispersed into single cells free in the culture. Sporoblasts accumulated to high densities in cultures, eventually giving rise to prespores, and then developing further into dinospores. Sporoblasts were morphologically similar to amoeboid trophonts, but perhaps more rounded than the amoeboid trophonts. When transferred into new medium, the sporoblasts were able to establish and initiate the development of new arachnoid trophonts rather than develop into dinospores.
Schizonts were embedded among the normal sporoblasts; however, they were much larger, being at least 3 times larger than the sporoblasts. As they progressed in their development, multiple cellular bodies formed within the schizonts. After a few weeks, the contents became motile and began writhing within the schizonts. When sporoblasts became detached from the arachnoid syncytium, the schizonts were also released into suspension. The motile cellular bodies within eventually broke through the schizont membrane and developed into aggregates of filamentous trophonts, which were morphologically similar to the gorgonlocks stage observed in cultures of Hematodinium Clade B parasites from the Norway lobster, N. norvegicus (Appleton and Vickerman, 1998). The gorgonlocks gradually separated into distinct filamentous trophonts, then underwent merogony (segmentation) to develop into aggregates of cellular clumps. When transferred into fresh medium, these clump colonies started forming syncytial networks, developing into arachnoid trophonts and then arachnoid sporonts.
In most successful cultures, the sporoblast stage developed further into a prespore – an apparent transition stage before developing into dinospores. This stage was occasionally observed in the circulating haemolymph of heavily infected crabs collected in the field. Prespores were not motile in haemolymph and cultures. Two types of dinospores, micro-dinospores or macro-dinospores, were observed in individual isolates, but they were never observed together in a single culture. Both spore types were actively motile in culture suspensions after the development of their flagella. Macro-dinospores were sluggish, typically moving along the substratum, while micro-dinospores were vigorous swimmers, easily moving throughout the culture medium.
Sexual reproduction occurs but the sexual stages remain undetermined (Pagenkopp et al. 2014).
Transmission: Hematodinium perezi is not effectively transmitted through ingestion of diseased tissues, indicating that cannibalism may not be a major route of transmission in blue crabs (Li et al. 2011). While the mode of transmission of the parasite to its hosts is unclear, it is thought that susceptible animals may become infected after moulting (Shields et al. 2005, 2007), suggesting an integumentary route of invasion when the cuticle is soft and vulnerable.
Shields JD, Taylor DM, Sutton SG, O’Keefe PG, Ings D, Pardy AL. Epidemiology of bitter crab disease
(Hematodinium sp.) in snow crabs Chionoecetes opilio from Newfoundland, Canada. Dis Aquat Org.
2005; 64: 253–264. PMID: 15997824
Shields JD, Taylor DM, O’Keefe PG, Colbourne E, Hynick E. Epidemiological determinants in outbreaks
of bitter crab disease (Hematodinium sp.) in snow crabs Chionoecetes opilio from Conception
Bay, Newfoundland, Canada. Dis Aquat Org. 2007; 77: 61–72. PMID: 17933398
Phases_alternance: haplontic
Symbiont: horizontal
Reproduction_mode: asexual
Reproduction_mode: sexual
Generation: <1 month