Amyloodinium ocellatum
Diagnosis
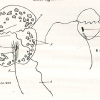
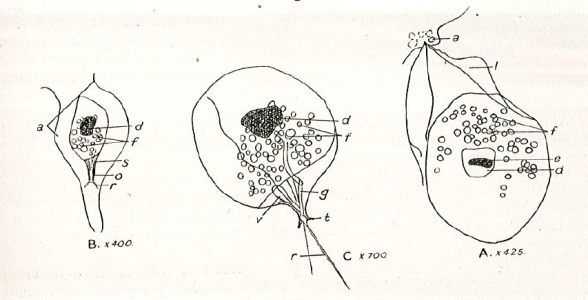
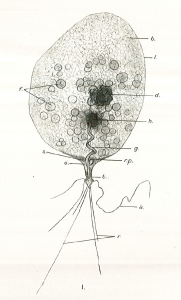
Diagnosis_Genus: Amyloodinium Brown & Hovasse 1946. Ectoparasites on gills but also on the rest of the surface of most various species of marine fishes. The trophont has an attachment disc with a very short peduncle, the circumference of the disc radiates into long filiform projections embedded deeply into the epithelial cells of the host. A special tentacle-like movable organ, stomopode, extends along with the peduncle of the attachment disc from the basal end of the cell: its function may by food ingestion and/or injection of lytic bodies into the host cells. No chloroplast. Thecal alveoli with plates. Cytoplasm contains large digestive vacuoles with particulate food, starch grains, splindle-shaped bodies, clove-like bodies and lytic bodies and subthecally located mucocysts and acontobolocysts. Size of trophont does not exceed 150 µm. Division of the palmella (tomont) stage takes place within a common cyst wall; up to 256 gymnospores produced. Gymnospore with stigma (eye spot).
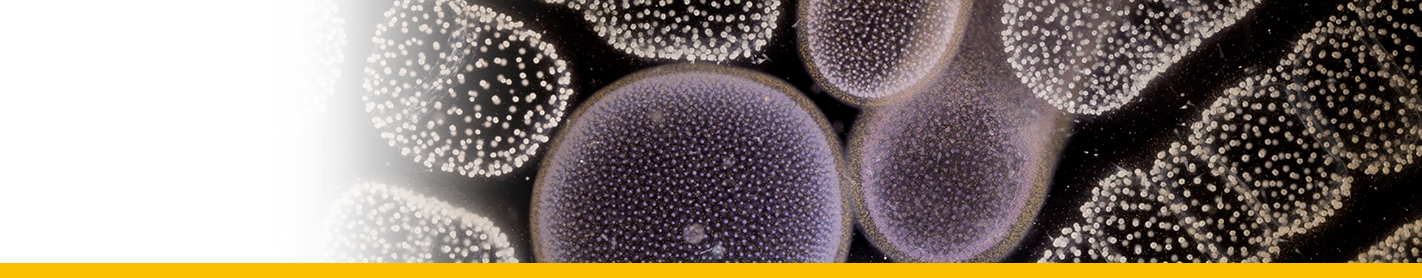
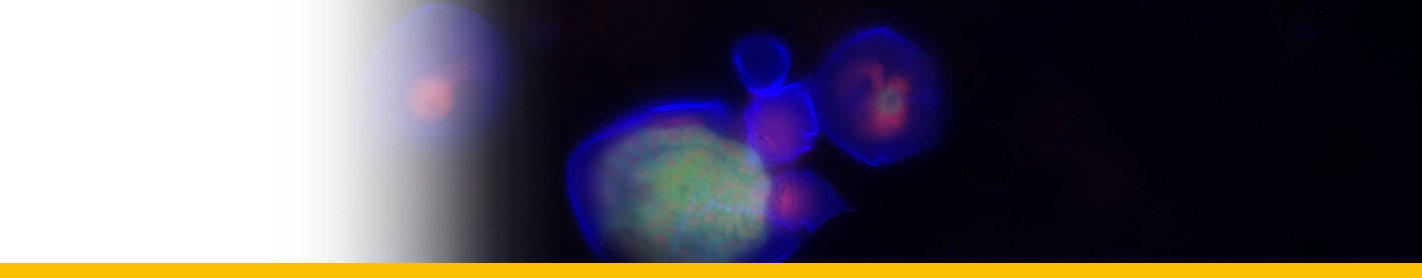
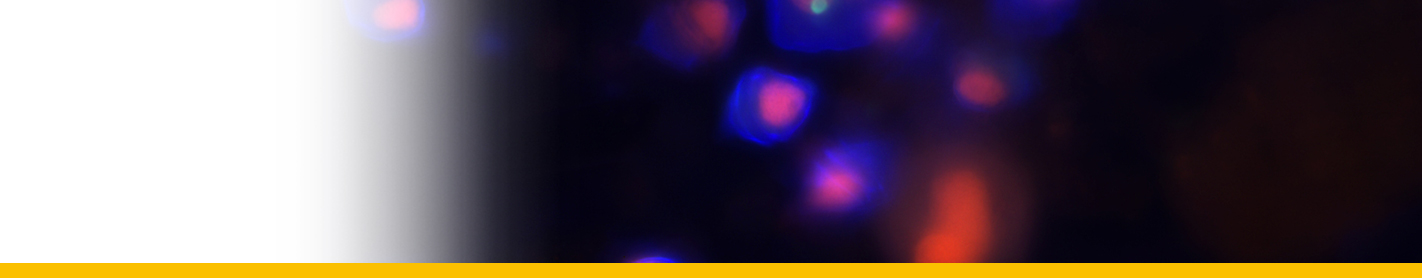
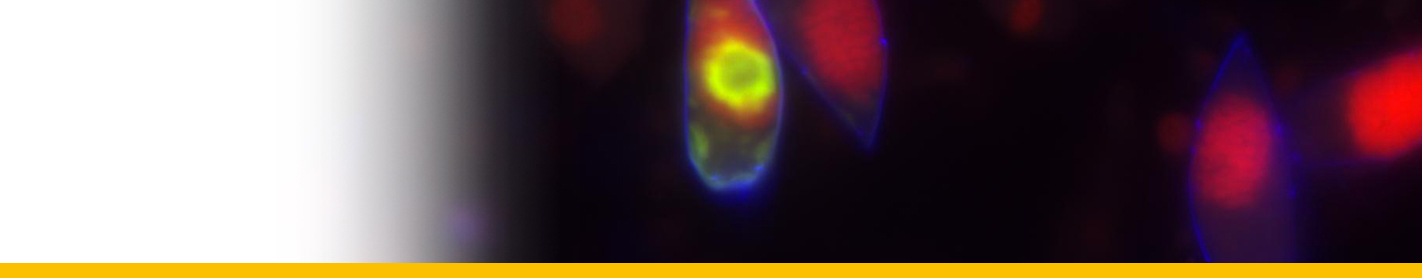
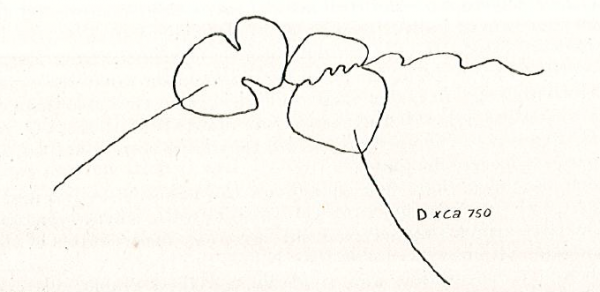
Diagnosis_Species: Amyloodinium ocellatum Brown 1931.Trophont spherical to oval (pear-shaped), average diameter 20-120 µm, attached to the gills by its narrow end, and are colourless and opaque. The parasite is attached to its host by means of a peduncle that ends by few rhizoids which penetrate the branchial tissues of the fish. A vesicular nucleus is central, with typical dinokaryon. An elongated red eyespot is visible in some trophont, but is difficult to observed owing to the density of the cell contents. The mature sporocyte can be readily detached from the host. Sporogenesis occurs outside the host only if temperature is suitable. Division is repeated giving rise to 4, 8, 16, 64 and 128 cells. Each daughter cells remains enclosed in the outer mother cuticule; but each new product of division secretes a new membrane. The successive membranes do not all appear to persist to the end of sporulation. Refringent granules distributed into daughter cells, without dividing, so their number is reduced at each successive divisions. The eyespot becomes more and more marked as division proceeds, and the cell contents become less opaque. At 128-cell stage, each cell divides to form two dinospores, which begin to move within the membrane and finally burst through it and swim away. The two spores are at first attached to each other at the anterior end, but soon become free and swim (typical dinoflagellate movement). Dinospores around 12.5-15 µm in length (Brown 1934). The eye-spot is present in the neighbourhood of the longitudinal flagellum. Dinospores have a plate pattern and tabulation of PO, cp, X, 4 ' , 1a , 7 " , 6 to 8?c, ?s, 5"', 2"", which are similar to that of the free-living Peridiniales (Landsberg et al. 1994).
A. ocellatum is the cause of heavy mortality among fishes in aquaria.
Infections can be successfully propagated on fish gill cell line (Noga 1987).
Body_trophont_length: 20-120 µm
Body_spores_length: 12.5-15 µm
Pigment: No
Etymology
The species name refers to the eyespot observed in the trophont, sporocyte and spores.
Type species
This is the type species of the genus.
Type illustration / Type locality / Type specimen
Type host: Fishes
Type locality: Warm marine fishes in the Society's Aquarium
Ecology
A. ocellatum can proliferate at a wide range of temperatures (23–41 ◦C) (Paperna, 1984; Noga, 2010) and salinities (3–46 psu) (Lawler, 1977; Kuperman and Matey, 1999).
Methods to manage A. ocellatum outbreaks include the use of chemical bath treatments such as copper sulfate, formalin, cloroquine diphosphate and hydrogen peroxide (Lawler, 1977; Paperna, 1984; Lewis et al., 1988; Montgomery-Brock et al., 2000, 2001; Noga and Levy, 2006), but these affect only the dinospore stage (Lawler, 1980; Paperna, 1984b). Other treatments include freshwater dips, ozone, UV sterilization and the experimental use of biological control agents such as wrasses, gobies or brine shrimps (Bower, 1987; Oestmann et al., 1995). In commercial-scale aquaculture, chemical bath treatments
are the most feasible treatments, but they typically only reduce the severity of infections, and repeated or long-term treatments are required (Blaylock and Whelan, 2004).
Substrate: epizoic
Sociability_trophont: gregarious
Salinity: marine
Salinity: brackish
Temperature: 23-41°C
Life cycle
Generation: <1 month
Reproduction_mode: asexual
Feeding behaviour
Mode of locomotion
Reference(s)
Observation site(s)
HOSTS
- ‹ previous
- 3 of 4
- next ›