Ichthyodinium chabelardi
Diagnosis
Diagnosis_Genus: Ichthyodinium Hollande and Cachon 1953. Endobiotic parasite of the early developmental stages of some species of finfish, particularly pelagic eggs and larvae in the ichthyoplankton. A complex live cycle of Ichthyodinium chaberladi was described by Hollande and Cachon 1953 on sardines from the Mediterranean Sea (Alger Bay), but complexity of the live cycle may differ depending on the parasite and/or the host species. First evidences of infection appear in the yolk sac of embryos after gastrulation (stages VI to XI, Dulcic 1998, Meneses et al. 2003). Infected eggs had no sign of penetration on its surface, suggesting that the parasite may pass through the hole for sperm penetration before the hole is completely closed (Yuasa et al. 2007).
Three successive stages of schizonts have been described by Hollande and Cachon 1953.
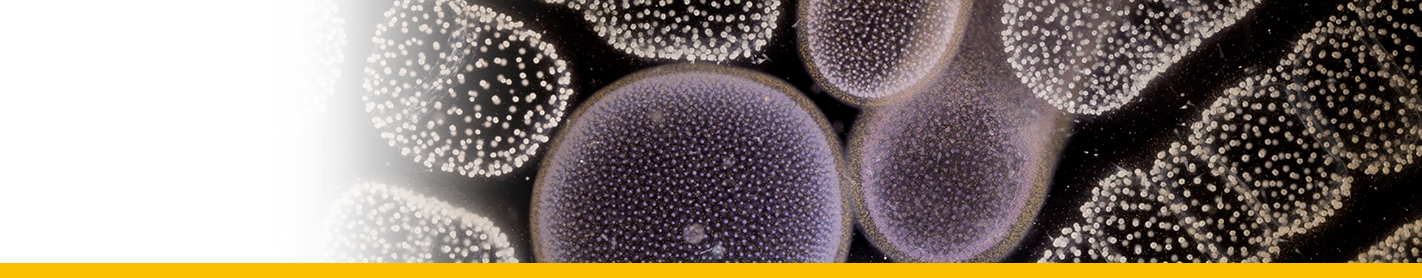
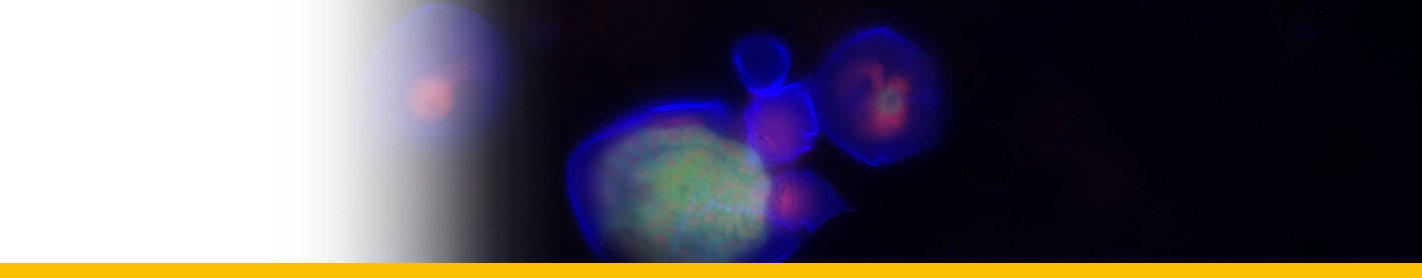
Primordial schizonts: The smallest stages of the parasite appear to be unicellular spheres (1 to 3 max per eggs) with a diameter of approximately 8-15 μm, less than 20 µm (Hollande and Cachon 1952, 1953, Meneses et al. 2003, Yuasa et al. 2007, Sorensen et al. 2014). These trophocytes absorb the vitellus material of the host in a central vacuole and remain uni-nucleated, although mitotic divisions following by transversal divisions may occur at this stage. Then, the nucleus undergoes multiple mitotic divisions without subsequent cytoplasmic division and the primordial schizont rapidly become large multinucleated structures of 100-140 μm. The cytoplasm forms cylindrical projections around each nucleus and each of this unit become a secondary schizont (~20-30 μm) that eventually separates from the rest in a budlike manner.
Secondary schizonts: They are cylindrical or in a racket-like shape, about 20 µm in length (Hollande and Cachon 1953). Lecithin starts to concentrate in these forms. This early secondary schizont first grows then starts to divide by longitudinal bipartitions. As the posterior poles of the two daughter cells remain attached, the parasites has successively a triangular form, ressembling to a rosace. Secondary schizonts then could eventually form a long cord (up to 1–2 mm) in layers of successive groups of eight cells connected by their poles (Hollande and Cachon 1953).
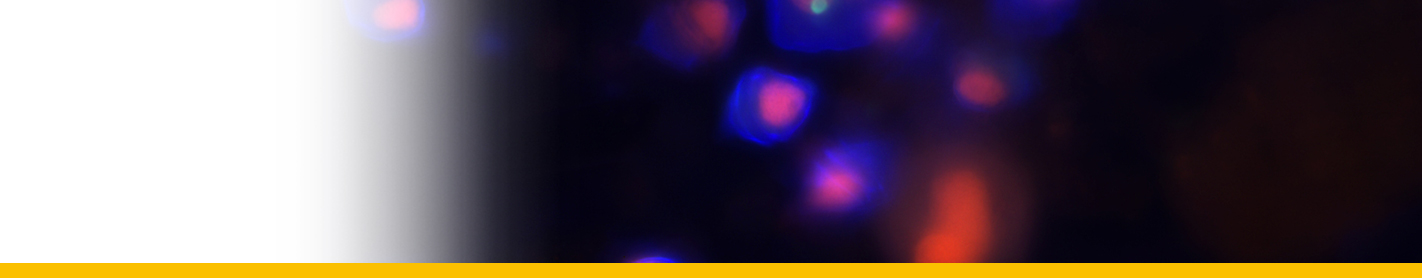
Last generation of schizonts: Oblong (cylindrical) schizonts are released from the cord, which become spherical after a series of divisions. At this phase, the yolk sac is entirely occupied by uni-nucleated parasitic cells or last generation of schizonts. This last generation of schizonts produce zoosporangia, which are released outside the host, in the water, generally after hatching. The yolk sac breaks causing the death of the newly hatched larvae. In some larvae, the parasite burst occurrs immediately after hatch, while it took more than 10 h after hatch in others (Mori et al. 2007). The number of sporangia released from a yolk-sac larva of the Yellowfin Tuna Thunnus albacares was estimated to be about 4 × 104 cells (Yuasa et al. 2007).
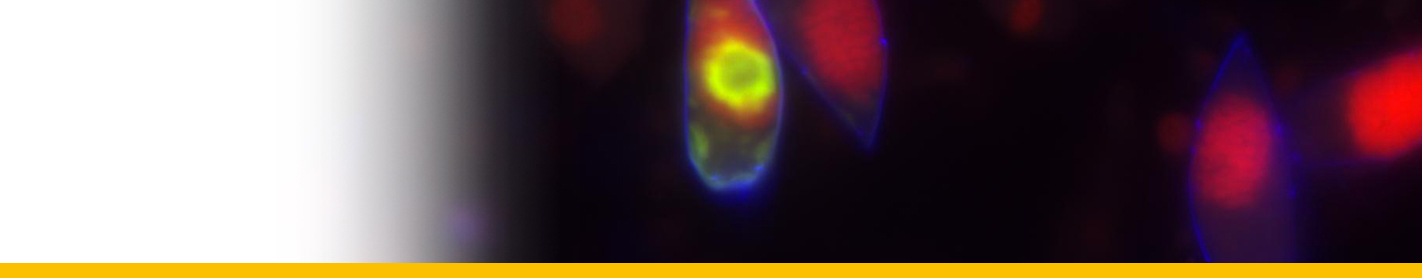
Spores: In the water, sporangia undergo one to two divisions and become flagellated (Hollande and Cachon 1953). These spores have trichocysts (Gestal et al. 2006), which are known to be involved in the host attachment in other Syndiniales (Miller et al. 2012). Nuclei of release spores exhibit numerous nuclear pores and have chromatin masses, permanently condensed in circular or oval profiles of DNA. In some cases, structures like rhoptrias and microtubule-organizing centres, similar to those characteristic of apicomplexan species, were observed at this developmental phase (Gestal et al. 2006). These structures may have similar function during the host invasion than dense bodies and striated strips observed in Amoebophrya (Miller et al. 2012). Spores remained actively swimming during few days, but then died (Hollande and cachon 1953). Spores of different sizes (Skovgaard et al. 2009) seem to correspond to different generations of division (Shadrin et al. 2015).
Variations observed from this complex live cycle: Three generations of schizonts, first described on sardines from the Mediterranean Sea was also observed in the mackerel off Portugal (Meneses et al. 2003). However, secondary schizonts seem to be absent on sardines off Portugal (Gestal et al. 2006, Borges et al. 1996), and several other host species, such as Plectropomus leopardus from Japan (Mori et al. 2007), and Thunnus albacore (Yuasa et al. 2007). In this case, primary schizonts lead directly to the last generation ones, without producing rosace and cord. No zoosporangium was formed in Plectropomus leopardus from Japan (Mori et al. 2007); instead, schizonts themselves transformed into zoospores within 10 min after release.
Diagnosis_Species: Ichthyodinium chaberladi Hollande and Cachon 1953. Two ribotypes (that may correspond to two different species) have be observed, separating up to now european from asiatic parasites. Additonal differences have been noticed depending on the host species.
Intensity of infections (number of parasitic individuals per host) is drastically lowest in eels (Sorensen et al. 2014), cods (Pedersen et al. 1993, Skovgaard et al., 2010), and turbot (Pedersen and Koie 1994). Infections not always lead to the dead of these hosts, and swarmer cells (spores) are not always produced, although bases of two flagella were found in the multicellular stage of the parasite in cod and in turbot (Pedersen and Koie 1994). Infections of these hosts appear to be considerably less effective, likely leading to a longer live cycle. Parasites were also observed in several tissues and bloodstream in the cod and the turbot, and have been even found in the beating heart (Pedersen & Køie 1994, Bloch et al. 1997). Recent data indicate that free-swimming I. chabelardi cells infect fish eggs after spawning (Mori et al. 2007, Yuasa et al. 2007), but it was previously suggested that this parasite infecting cods and turbots are transferred vertically through the fish gametes (Pedersen & Køie 1994), which may explain why swarmers have not been detected, why the parasite extended invasion to other tissues, and why the infectivity/aggressiveness was reduced in these hosts.
Type species
This is the type species of the genus.
Type illustration / Type locality / Type specimen
Type host: Sardina pilchardus and Maurolicus pennanti.
Type locality: Alger Bay
Ecology
Substrate_trophont: endozoic
Substrate_spores: planktonic
Salinity: marine
pH: neutral
Life cycle
Generation: <1 month
Reproduction_mode: asexual
Symbiont: horizontal